Energy Changes
Energy is the ability to do work. It exists in several forms such as heat, light, sound, electrical, potential (stored), and kinetic energy.
Law of Conservation of Energy
Energy can be converted from one form to another, but the total amount remains unchanged. This principle is called the law of conservation of energy. It states:
Energy can neither be created nor destroyed, but it can be transformed from one form to another.
Types of Energy
Some common types include:
- Chemical energy
- Heat energy
- Light energy
Heat Content (Enthalpy) of a Substance
Enthalpy is the internal energy of a substance due to its structure and physical state. Potential energy comes from structure, while kinetic energy depends on its state. We can’t measure total enthalpy, only the change in enthalpy (\( \Delta H \)).
Enthalpy change: \( \Delta H = H_{\text{products}} - H_{\text{reactants}} \)
This value can be either positive or negative and is written alongside the chemical equation.
Example:
\( \text{HCl}_{(aq)} + \text{NaOH}_{(aq)} \rightarrow
\text{NaCl}_{(aq)} + \text{H}_2\text{O}_{(l)} \)
\(
\Delta H = -57.3\, \text{kJ} \)
Unit: Joules (J) or kilojoules (kJ)
Exothermic and Endothermic Reactions
Exothermic Reactions
In exothermic reactions, heat is released to the surroundings. The temperature increases and the container feels hot.
Examples:
- Calcium oxide + water
- Acid-base neutralization
- Combustion of fuels
- Metal corrosion
- Respiration
Enthalpy change: Negative, since \( H_{\text{products}} < H_{\text{reactants}} \)
Endothermic Reactions
Endothermic reactions absorb heat from the surroundings. The temperature drops and the container feels cold.
Examples:
- Thermal decomposition of calcium carbonate
- Thermal dissociation of ammonium chloride
- Light action on silver bromide in photography
- Photosynthesis
Enthalpy change: Positive, since \( H_{\text{products}} > H_{\text{reactants}} \)
Energy Level Diagrams
Energy level diagrams show whether a reaction is exothermic or endothermic by comparing the energy of reactants and products.
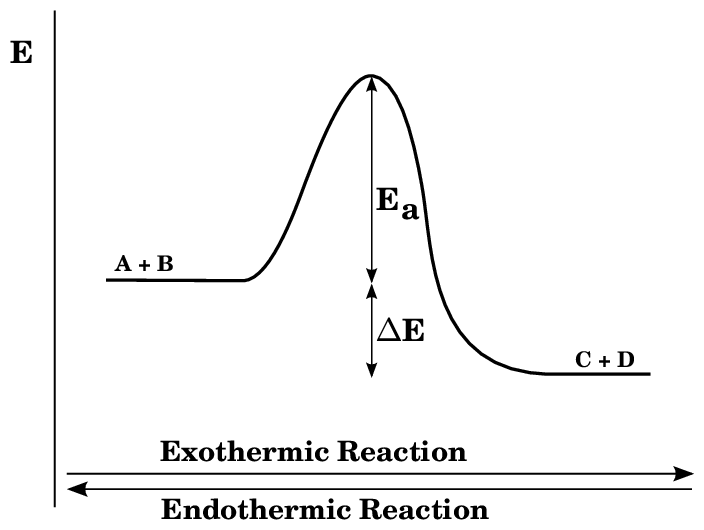 Credit: Sergio Ioppolo on researchgate
Credit: Sergio Ioppolo on researchgate
Heat of Reaction and Chemical Bonds
During chemical reactions:
- Bond breaking requires energy (endothermic)
- Bond forming releases energy (exothermic)
The minimum energy needed to start breaking bonds is called activation energy.
If bond-breaking energy < bond-forming energy →
reaction is exothermic
If bond-breaking energy > bond-forming energy →
reaction is endothermic
Types of Heat Changes
1. Heat of Formation
The heat change when 1 mole of a compound is formed from its elements under standard conditions.
Example:
\( \text{H}_2(g) + \frac{1}{2}\text{O}_2(g) \rightarrow
\text{H}_2\text{O}(l) \)
\( \Delta H_f^\theta = -285\, \text{kJ mol}^{-1} \)
2. Heat of Neutralization
The heat released when 1 mole of H+ reacts with 1 mole of OH− to form water.
Reaction:
\( \text{H}^+(aq) + \text{OH}^-(aq) \rightarrow
\text{H}_2\text{O}(l) \)
\( \Delta H_n^\theta = -57.4\, \text{kJ mol}^{-1} \)
3. Heat of Combustion
The heat released when 1 mole of a substance is completely burned in oxygen under standard conditions.
Formula:
\( \text{Heat of combustion} = \frac{\text{Heat produced} \times \text{Molar mass}}{\text{Mass burnt}} \)
If water is used to absorb the heat:
\( \text{Heat of combustion} = \frac{mC\Delta\theta \times \text{Molar mass}}{\text{Mass burnt}} \)
Where:
- \( m \) = mass of water
- \( C \) = specific heat capacity of water
- \( \Delta \theta = \theta_2 - \theta_1 \) (temperature change)
4. Heat of Solution
The heat change when 1 mole of a substance dissolves in excess water such that no further heat change occurs on further dilution.
This heat change can be exothermic or endothermic.
Thermodynamics
Thermodynamics studies the relationship between heat and other forms of energy.
System and Surroundings
A system is any part of the universe under study, which can be:
- Open system - exchanges matter and energy
- Closed system - exchanges only energy
- Isolated system - exchanges neither
The surrounding is everything outside the system.
First Law of Thermodynamics
Energy cannot be created or destroyed, only converted from one form to another.
Internal Energy Change
Internal energy (\( U \)) changes when:
- Heat (\( q \)) is absorbed or released
- Work (\( w \)) is done by or on the system
Mathematically: \( \Delta U = q + w \)
Note: Work done by the system is negative (it reduces internal energy).
First Law of Thermodynamics
The first law of thermodynamics states that energy cannot be created or destroyed; it can only be transformed from one form to another.
Mathematical Expression
For a system, the change in internal energy is given
by:
$$ \Delta U = q - w $$
For a gaseous system where work w is done by expansion against pressure: $$ w = P \Delta V $$ Therefore: $$ \Delta U = q - P \Delta V $$
Enthalpy (H) is related to internal energy (U) as: $$ H = U + P \Delta V $$ Rearranging: $$ \Delta U = H - P \Delta V $$
Second Law of Thermodynamics
The second law of thermodynamics states that a spontaneous process can occur only if the total entropy of the system and its surroundings increases.
Factors Affecting Spontaneity
- Enthalpy (H): The heat content of the substances involved in the reaction.
- Entropy (S): A measure of the randomness or disorder of a system.
- Free Energy (G): The energy available to do work in a system.
Entropy (S)
Entropy, \( S \), is a measure of the degree of disorder in a system. The standard entropy change is:
$$ \Delta S^\theta = S^\theta_{\text{products}} - S^\theta_{\text{reactants}} $$
The SI unit of entropy is J·K-1·mol-1.
Entropy increases as substances change from solid → liquid → gas due to increased randomness.
For a reversible process at constant temperature: $$ \Delta S = \frac{\Delta H}{T} $$
- If \( \Delta S \) is positive, entropy increases.
- If \( \Delta S \) is negative, entropy decreases.
Gibbs Free Energy (G)
The free energy, \( G \), is the energy available to do useful work. The change in free energy at constant temperature is given by:
$$ \Delta G^\theta = \Delta H^\theta - T \Delta S^\theta $$
Interpretation of ΔG
- If \( \Delta G < 0 \), the reaction is spontaneous.
- If \( \Delta G > 0 \), the reaction is non-spontaneous unless compensated by changes in enthalpy and entropy.
- If \( \Delta G = 0 \), the system is at equilibrium.
Example
Consider the reaction:
C(s) + O2(g) →
CO2(g)
Given:
- Temperature: 57°C = 330 K
- ΔH = -5000 J
- ΔS = +15 J/K
Calculate the change in free energy, \( \Delta G \):
$$ \Delta G = \Delta H - T \Delta S
$$
$$ \Delta G = -5000 - (330 \times 15)
$$
$$ \Delta G = -5000 - 4950 $$
$$ \Delta G = -9950 \text{ J} \text{ or }
-9.95 \text{ kJ} $$